Abstract
Background: An inflammatory bone marrow microenvironment with unchecked immune activation mediated by cytotoxic T cells and defective T regulatory cells contribute to acquired bone marrow failure (BMF) syndromes, including aplastic anemia (AA), myelofibrosis (MF) and hypoplastic myelodysplasia (MDS). Adoptive therapy with allogeneic cord blood (CB) T-regulatory (Treg) cells has shown safety in humans and clinical benefit in graft vs. host disease. Derived from CB, these Treg cells enjoy immune privilege, demonstrate high proliferative index, lack plasticity, and have been shown to consistently suppress inflammation. Therefore, we hypothesized that adoptive therapy with CB Tregs can be utilized as treatment for the inflammatory BMF disorders.
Study Design and Methods: This is a phase I clinical trial examining the role of a single infusion of CK0801, an allogeneic, fresh CB Treg product for the treatment of BMF. CK0801 was produced utilizing novel process development that consists of well-defined qualification criteria for the starting material (CB units), parameters for manufacturing and culture-expansion; and well-defined analytic testing and lot release criteria. Three dose-levels were examined: i) 1x10 6 cells/kg; ii) 3x10 6 cells/kg and iii) 10x10 6 cells/kg (NCT03773393). No immune suppression or lymphodepletion was administered. Patients were allowed to continue their ongoing treatment at stable doses. The study followed a 3+3 phase dose-escalation design. Patients aged ≥ 18 yrs with AA, MDS or MF, and available (HLA 3 out of 6) matched CB units were eligible. The primary endpoint is to determine the maximum tolerated dose (MTD) and dose limiting toxicity (DLT) of the Treg infusion defined as any of the following 3 events: i) severe (grade 3 or 4) infusion toxicity within 24 hours (NCI-CTCAE V4.0), ii) regimen related death within 30 days, or iii) severe (grade 3 or 4) cytokine release syndrome (CRS) within 30 days. Secondary endpoints include exploration of efficacy, PB and BM immune reconstitution, and inflammatory cytokines.
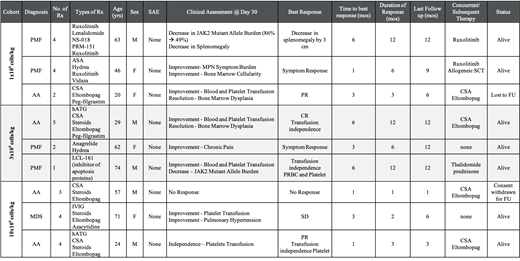
Results: Nine patients (Pts) have been treated, with a median age of 57 (range, 20-74) with a diagnosis of MF (n=4); AA (N=4) and hypoplastic MDS (n=1). Overall, the patients had received a median of 4 prior therapies (range, 1-5). There were no infusion related reactions and no DLTs in any of the patients. The patient characteristics and day 30 response are summarized in Table 1. The median time to best response was 3 months (range, 1-6) with complete response (n=1); partial response (n=2); symptom response (n=2); anemia response (n=1); stable disease (n=2) and no response (n=1) (Figure 1). The median duration of response was 6 months (range, 1-12). Of the 5 transfusion dependent pts, transfusion independence was reported in 3 pts (AA = 2; MF= 1). Improvement in transfusion requirement was seen in 2 pts (AA=1; hypoplastic MDS = 1). All AA pts continued their immunosuppressive therapies and the 2 MF patients continued their Ruxolitinib. Decrease in inflammatory biomarker levels as well as lower Th17 cells were observed post CK0801 infusion. No impact on the anti-viral activity of the normal CD4+ and CD8+ T cells was observed (data will be presented later).
Conclusion: A single infusion of allogeneic CB derived Treg cells (CK0801) is well tolerated, feasible, and may be associated with clinical improvement in patients with immune related bone marrow disorders. Clinical response appears to correlate with a decrease in inflammatory biomarkers. Expansion of the study, including multiple repeated Treg dosing in BMF is planned.
Kadia: Jazz: Consultancy; Pfizer: Consultancy, Other; Pulmotech: Other; Sanofi-Aventis: Consultancy; Cellonkos: Other; Ascentage: Other; Genfleet: Other; Astellas: Other; AstraZeneca: Other; Novartis: Consultancy; Liberum: Consultancy; AbbVie: Consultancy, Other: Grant/research support; Aglos: Consultancy; Genentech: Consultancy, Other: Grant/research support; Dalichi Sankyo: Consultancy; Cure: Speakers Bureau; BMS: Other: Grant/research support; Amgen: Other: Grant/research support. Pemmaraju: Blueprint Medicines: Consultancy; Clearview Healthcare Partners: Consultancy; CareDx, Inc.: Consultancy; Stemline Therapeutics, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Sager Strong Foundation: Other; Protagonist Therapeutics, Inc.: Consultancy; Cellectis S.A. ADR: Other, Research Funding; Daiichi Sankyo, Inc.: Other, Research Funding; Affymetrix: Consultancy, Research Funding; Incyte: Consultancy; Novartis Pharmaceuticals: Consultancy, Other: Research Support, Research Funding; LFB Biotechnologies: Consultancy; Celgene Corporation: Consultancy; Abbvie Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; MustangBio: Consultancy, Other; Roche Diagnostics: Consultancy; DAVA Oncology: Consultancy; Springer Science + Business Media: Other; Aptitude Health: Consultancy; Plexxicon: Other, Research Funding; HemOnc Times/Oncology Times: Membership on an entity's Board of Directors or advisory committees; ASCO Leukemia Advisory Panel: Membership on an entity's Board of Directors or advisory committees; Dan's House of Hope: Membership on an entity's Board of Directors or advisory committees; ASH Communications Committee: Membership on an entity's Board of Directors or advisory committees; Samus: Other, Research Funding; Bristol-Myers Squibb Co.: Consultancy; ImmunoGen, Inc: Consultancy; Pacylex Pharmaceuticals: Consultancy. Yilmaz: Daiichi-Sankyo: Research Funding; Pfizer: Research Funding. Daver: Novartis: Consultancy; Trovagene: Consultancy, Research Funding; Trillium: Consultancy, Research Funding; Sevier: Consultancy, Research Funding; Gilead Sciences, Inc.: Consultancy, Research Funding; Astellas: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; FATE Therapeutics: Research Funding; ImmunoGen: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Hanmi: Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Glycomimetics: Research Funding; Novimmune: Research Funding; Jazz Pharmaceuticals: Consultancy, Other: Data Monitoring Committee member; Dava Oncology (Arog): Consultancy; Celgene: Consultancy; Syndax: Consultancy; Shattuck Labs: Consultancy; Agios: Consultancy; Kite Pharmaceuticals: Consultancy; SOBI: Consultancy; STAR Therapeutics: Consultancy; Karyopharm: Research Funding; Newave: Research Funding. Parmar: Cellenkos Inc.: Current holder of individual stocks in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding. Mukherjee: Vor Biopharma: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties: coinventor on issued and pending patent applications licensed to Vor Biopharma. S.M. has equity ownership and is on the Scientific Advisory Board of Vor Biopharma., Research Funding. Sadeghi: Cellenkos Inc.: Current Employment. DiNardo: Takeda: Honoraria; Notable Labs: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Forma: Honoraria, Research Funding; ImmuneOnc: Honoraria, Research Funding; AbbVie: Consultancy, Research Funding; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria; Agios/Servier: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; Celgene, a Bristol Myers Squibb company: Honoraria, Research Funding; Foghorn: Honoraria, Research Funding. Issa: Novartis: Consultancy, Research Funding; Syndax Pharmaceuticals: Research Funding; Kura Oncology: Consultancy, Research Funding. Jabbour: Amgen, AbbVie, Spectrum, BMS, Takeda, Pfizer, Adaptive, Genentech: Research Funding. Borthakur: Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Astex: Research Funding; ArgenX: Membership on an entity's Board of Directors or advisory committees; Ryvu: Research Funding; University of Texas MD Anderson Cancer Center: Current Employment; Protagonist: Consultancy; GSK: Consultancy. Jain: Bristol Myers Squibb: Honoraria, Research Funding; Cellectis: Honoraria, Research Funding; Beigene: Honoraria; AstraZeneca: Honoraria, Research Funding; Incyte: Research Funding; Aprea Therapeutics: Research Funding; Servier: Honoraria, Research Funding; Adaptive Biotechnologies: Honoraria, Research Funding; Pfizer: Research Funding; TG Therapeutics: Honoraria; Janssen: Honoraria; Fate Therapeutics: Research Funding; Precision Biosciences: Honoraria, Research Funding; ADC Therapeutics: Honoraria, Research Funding; Genentech: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Pharmacyclics: Research Funding. Verstovsek: Celgene: Consultancy, Research Funding; Gilead: Research Funding; Incyte Corporation: Consultancy, Research Funding; NS Pharma: Research Funding; Ital Pharma: Research Funding; Roche: Research Funding; CTI BioPharma: Research Funding; Genentech: Research Funding; Protagonist Therapeutics: Research Funding; Promedior: Research Funding; PharmaEssentia: Research Funding; Blueprint Medicines Corp: Research Funding; AstraZeneca: Research Funding; Novartis: Consultancy, Research Funding; Sierra Oncology: Consultancy, Research Funding; Constellation: Consultancy; Pragmatist: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal